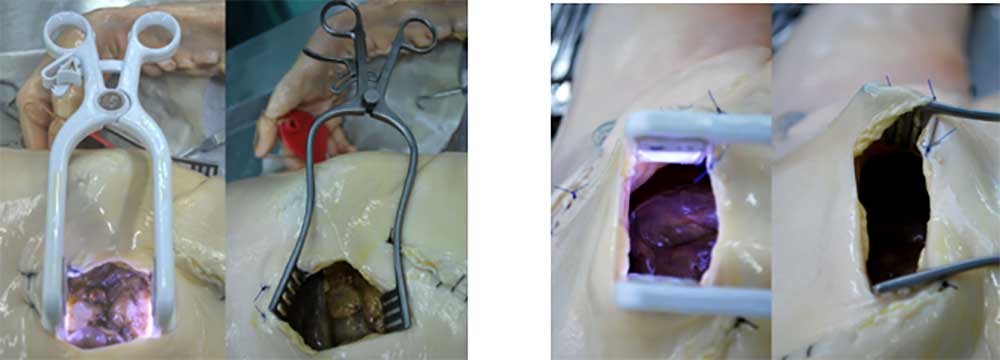
This illuminated retractor combines a conventional self retaining retractor with the addition of an in-built cold light source. The purpose of this ‘Cold Light’ Illuminated Retractor is to provide surgeons with a light source which directly illuminates the interior cavity of an incision during surgery while the retractor holds open the incision. Ultimately this provides two functions in a single device. The device is a Class IIa medical device (sterile).
Typically surgeons will illuminate the operating field using overhead lamps, headlamps, fibre optic cables or even on some occasions a torch has been used. The preferred method is a fibre optic cable attached to a retraction system. This is neither ergonomic nor optimal as the light produces shadows within the cavity and may not reach the deepest areas. Also, fibre optic cable can impede the free movement of staff around the operating table. The aim of the Oplight technology is to simplify the necessary equipment and improve illumination of the surgical site.
The retractor design and development is almost complete; it is made from biocompatible plastic and uses LED technology to illuminate the surgical cavity. The Oplight will be a disposable/single patient use device thereby eliminating any possibility of cross contamination. The light source has a controllable On/Off switch with up to 4 hours of illumination possible.
Fearsomengine Ltd,
752-756 Argyle Street, Glasgow, G3 8UJ
Tel: 0141 248 3224
EUROPLAZ TECHNOLOGIES LIMITED
Unit 1, The Maltings Industrial Estate,
Southminster, Essex, CM0 7EH
Tel: 01621 773471
Andersen Caledonia Ltd
Phoenix Crescent
Strathclyde Business Park
Bellshill
ML4 3NJ
Tel: 01698 844476
Design LED Products
Alba Innovation Centre
Livingston
EH54 7GA, UK
Tel: 01506 592310, Fax. +44 (0)1506 592311